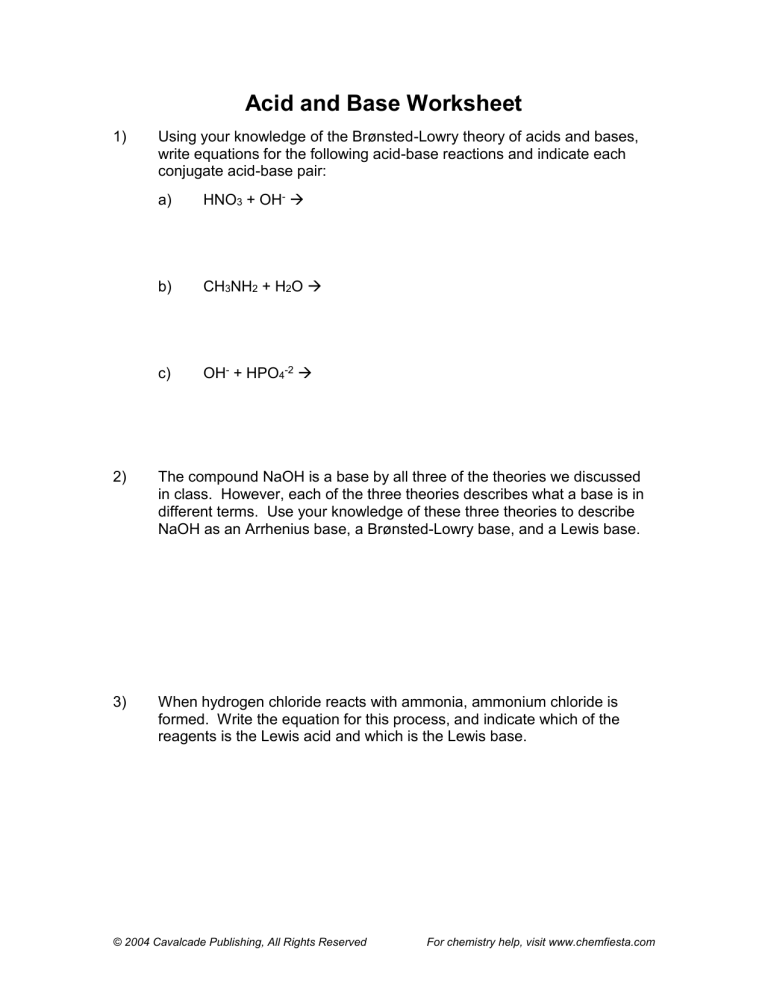
Which Best Describes the Definition of Lewis Acids and Bases
The difference is context-specific and varies based on the reaction. The hydrogen atom is not included in this theorys description of acids and bases.
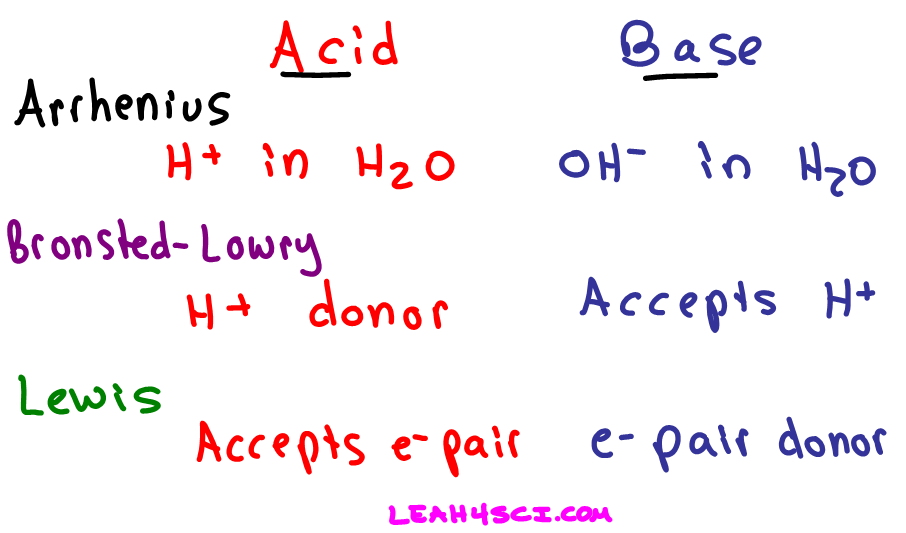
Definitions Of Arrhenius Bronsted Lowry And Lewis Acids And Bases In Organic Chemistry
Iron Fe 2 and Fe 3.

. According to the Lewis definition acids are molecules or ions capable of coordinating with unshared electron pairs and bases are molecules or ions having unshared electron pairs available for sharing with acids. Think of Lewis as lectrons Lewis Acid. See answers 2 Best Answer.
Da specific definition based on a compounds ability to donate protons. In other words a Lewis acid is an electron-pair acceptor. A Lewis base is any substance such as the OH-ion that can donate a pair of nonbonding electrons.
Lewis bases have occupied relatively high energy atomic or molecular orbitals. A Lewis base is a type of species that can donate a pair of electrons to the acceptor of the same category. A Lewis acid is an electron -pair acceptor.
THIS IS THE BEST ANSWER Lewis acid is an electron-pair receiver and Lewis base is an electron-pair donor. Which best describes the definition of lewis acids and bases. A Lewis acid refers to an atom or molecule that accepts an electron pair.
Instead the Lewis definition deals with the movement of electrons. Bases can be thought of as the chemical opposite of acids. The Lewis definition for acids and bases is the most extreme because its not dealing with protons specifically.
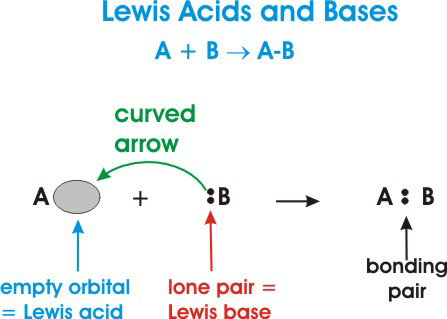
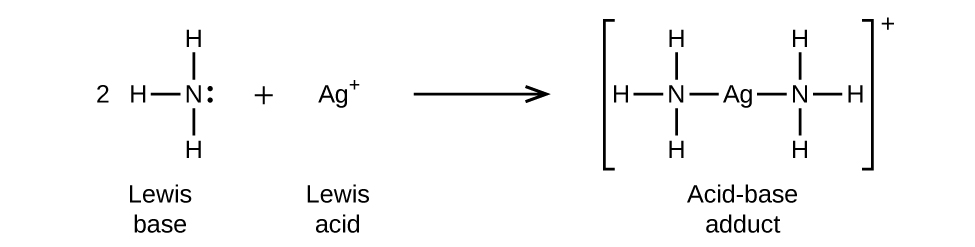
They can react with each another such that a covalent bond forms with both electrons provided by the Lewis base. A Lewis acid is an electron pair acceptor and a Lewis base is an electron pair donor. For example NH3 is a Lewis base.
Ba general definition based on hydrogen ion concentration. A substance that accepts electrons to form a covalent bond. Arrhenius definition of a base.
Any species that can accept react with a proton including but not limited to the OH are bases. The reaction of Lewis acidbase forms a bond that is known as a coordinate covalent bond. Lewis Concept of Acids and Bases.
A Lewis base is a species that has a single pair of electrons and hence can operate as an electron donor. The third scientist to define an acid and base was Lewis. A Lewis base is a chemical species having the full orbital and has a pair of electrons that are not in the bond and can form a dative bond with a Lewis acid.
Gilbert Newton Lewis same Lewis who is behind the electron-dot formulas suggested even a more general way of classifying acids and bases. An acid according to Lewis definition is a species with an empty orbital and hence the ability to take an electron pair. According to Lewis acids are the species that are able to accept an electron pair from a base or electron donating specie.
A fact is something that is true and you have information to back it up. 2018-02-05 12. Lewis acids have an unoccupied low-energy atomic or molecular orbital.
Lewis Acids and Bases. In the Lewis theory of acid-base reactions bases donate pairs of electrons and acids accept pairs of electrons. Which best describes the definition of.
HF H2O - H3O F-. Click card to see definition. For example Me3B trimethylborane A Lewis base is an element or compound capable of donating a pair of electrons.
What definition best describes a base. Consider the reaction below. For example is a lewis acid as it is able to accept an electron.
It also explains the concept of a conjugate pair - an acid and its conjugate base or a base and its conjugate acid. This is a more flexible. Tap again to see term.
A Lewis base is an electron pair donor. An acid is a substance that donates protons in the Brønsted-Lowry definition or accepts a pair of valence electrons to form a bond in the Lewis definition. His definition was that an acid is a substance that accepts an electron pair to form a covalent bond and a base is a substance that.
Many reactions involve Lewis acids and bases. Cations of d block elements that. Lewis Acids and bases.
A base is a substance in a solution that captures hydrogen ions and raises the pH. Some of the common examples are. Which is an acid-conjugate base pair.
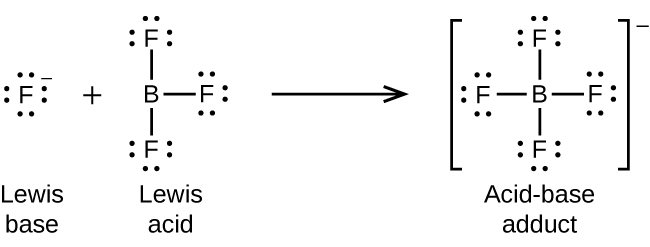
Aa general definition based on electron structure. A substance in a solution that releases hydrogen ions and lowers the pH. A Lewis acid is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct.
A Lewis acid is an element or compound that has space in the last orbital to accept a pair of electrons. The acid-base reaction definition describes the chemical change that occurs in a reaction between acid and base. What is the definition of a Lewis acid.
According to the Lowry-Bronsted definition an acid is a proton donor and a base is a proton acceptor. Ca specific definition based on a compounds ability to accept protons. A base is a substance that can accept protons or donate a pair of valence electrons to form a bond.
A Lewis acid is therefore any substance such as the H ion that can accept a pair of nonbonding electrons. A Lewis base then is any species that has a filled orbital containing an electron pair which is not involved in bonding but may form a dative bond with a Lewis acid to form a Lewis adduct. Some of the Examples of Lewis Acids.
Some molecules can act as either Lewis acids or Lewis bases. An acid is a substance that donates H ions in a reaction. A Lewis base is an electron-pair donor.
A Lewis acid is an electron pair acceptor. Which best describes the definition of Lewis acids and bases. Lewis acids and bases result in the formation of an adduct rather than a simple displacement reaction as with classical acids and bases.
The base reaction with a proton donor an acid leads to the exchange of protons. This page describes the Arrhenius Bronsted-Lowry and Lewis theories of acids and bases and explains the relationships between them.
Chapter 12 Acid Base Chemistry

Lewis Acids And Bases Wikiwand
15 2 Lewis Acids And Bases Chemistry
15 2 Lewis Acids And Bases Chemistry
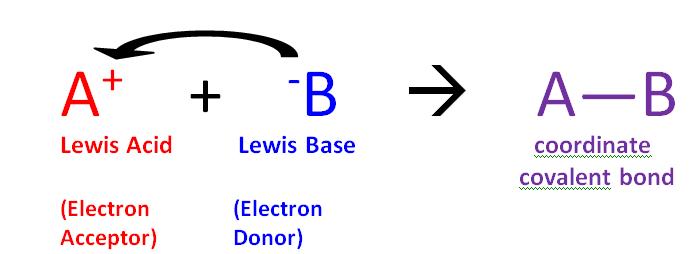
16 7 Lewis Concept Of Acids And Bases Chemistry Libretexts

Lesson Video Lewis Acids And Bases Nagwa

Solved Definitions And Introduction To Lewis Acids And Bases Chegg Com

Reversible And Irreversible Acid Base Reactions In Organic Chemistry
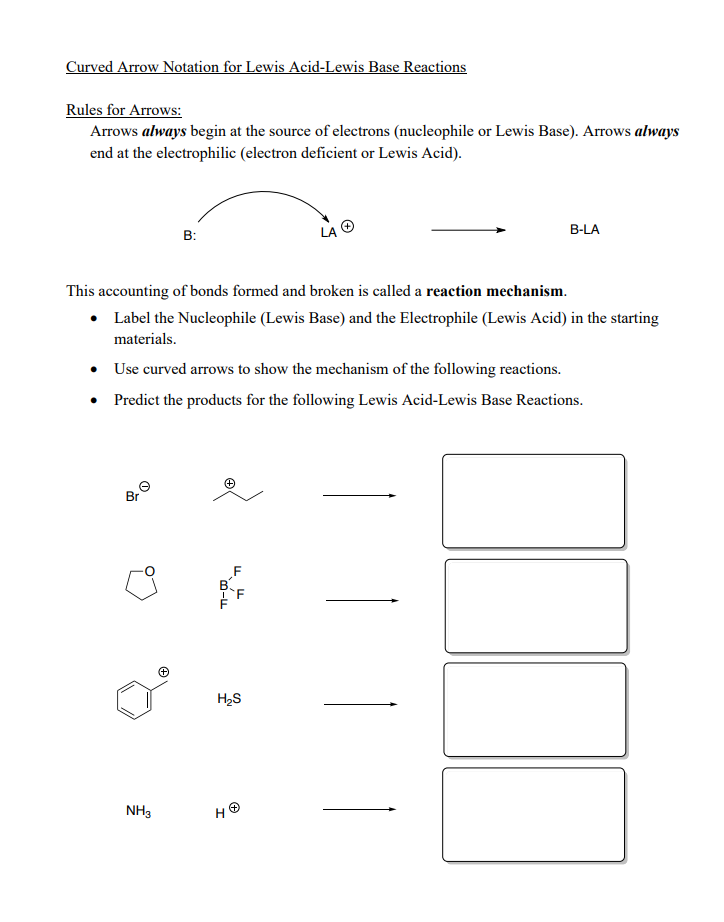
Solved Definitions And Introduction To Lewis Acids And Bases Chegg Com

Relative Lewis Acid Base Potential On An Arbitrary Scale Within Each Download Scientific Diagram
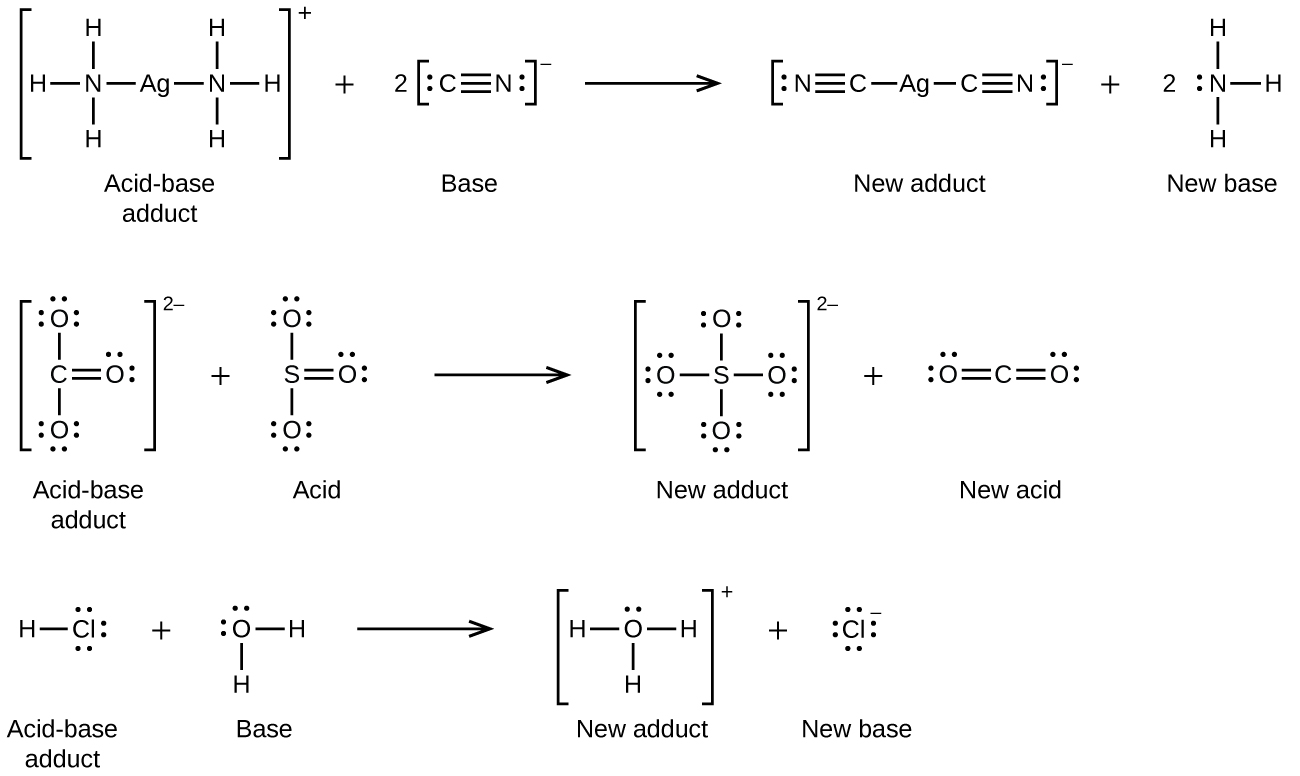
15 2 Lewis Acids And Bases Chemistry

Lewis Acids And Bases Wikiwand

The Lewis Definitions Of Acids And Bases

Lewis Acids And Bases Ck 12 Foundation

Lesson Explainer Lewis Acids And Bases Nagwa
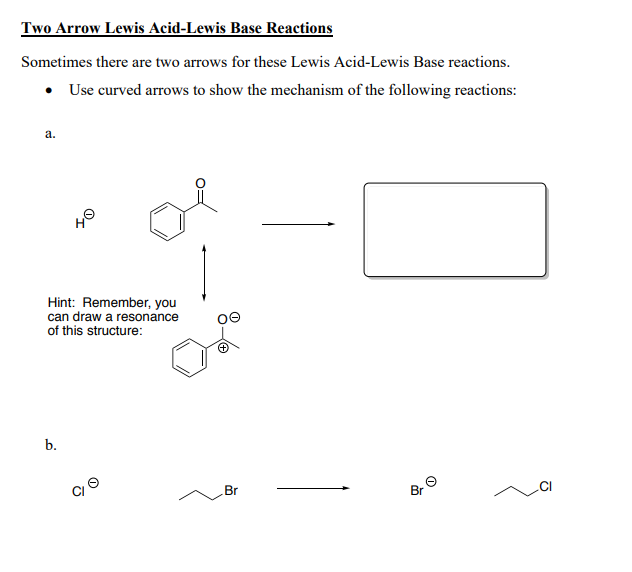
Solved Definitions And Introduction To Lewis Acids And Bases Chegg Com

Comments
Post a Comment